-
Nickel Periodic Table카테고리 없음 2021. 7. 1. 00:10

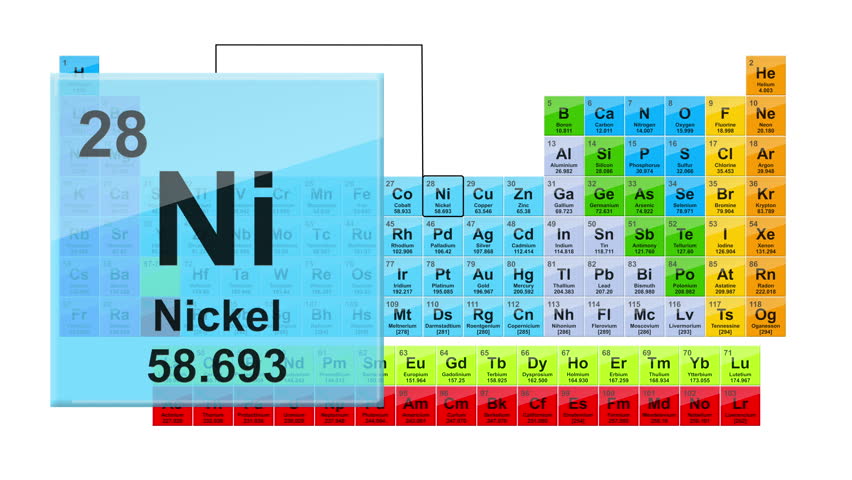
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. Each of us has a good memory of the school period. The periodic table of the elements was certainly a part of our journey. Therefore it is easy to check that Nickel is placed in the fourth period which means it has four energy levels and in the category of transition metals. This can be seen in the periodic table at the top of the page. As seen on the periodic table, Nickel is a transition metal and as Cronstedt discovered it is white in colour. Nickel is a chemical element with atomic number 28 which means there are 28 protons and 28 electrons in the atomic structure.
PREVIOUS | NEXT

Porcupine Plain Composite High School, Porcupine Plain, Saskatchewan, Canada
Nickel: For the symbol, we used a magnet and a battery because nickel is found in both. The green background and the green atomic number represent that nickel is used as a tint to make glass green. The devil represents “devil’s copper”, which nickel was referred to because it interfered with the smelting of copper. Nickel is found in many meteorites. Lastly, the 5 cent coin (commonly called the “nickel”), was previously made mostly out of nickel but is now only partly made out of nickel.
Jason Harbor and the last Chemistry 30 class at Porcupine Plain Composite High School, Porcupine Plain, Saskatchewan, Canada
Atomic properties*
 Nickel
NickelExplanation Of The Periodic Table
Ni2858.69 amu1455°C2913°C8.9 g/cm31.917SolidLustrous, metallic-silvery-tinge±Metallic±* Haynes, W. M. (2011). CRC Handbook of Chemistry and Physics, 91st edition: http://www.hbcponline.com/ Retrieved April 7, 2011
± Winter, M. (2010). Home of the Periodic Table. Retrieved April 8, 2011, from Web Elements: http://www.webelements.com/
What is the noble gas notation of nickel?
1 Answer
Nickel (Ni) is located in the 4th energy level (row) of the periodic table, column 10 (VIII). This position would be an electron configuration of
#1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^7# Nickel Periodic Table
To convert this to the Noble Gas notation we revert back to the Noble Gas from period 3 of the periodic table Argon
Nickel Periodic Table Square

Argon - Ar
#1s^2 2s^2 2p^6 3s^2 3p^6# We then replace this section of Nickel's electron configuration with [Ar].
#[Ar] 4s^2 3d^7# I hope this was helpful.
SMARTERTEACHERRelated questions
